Manteia Predictive Medicine

Why was Manteia Important?
There are three main components to the sequencing approach used by Illumina, originally developed by Solexa in 2004:
- Sequencing-by-synthesis (detection of nucleotides incorporated by a polymerase)
- Reversibly terminated, labelled nucleotides
- Cluster generation through bridge amplification.
The general concept of sequencing-by-synthesis had been around since at least 1989.
Reversible terminators, in particular the 3′-O-Azidomethyl terminators used by Solexa were originally reported in 1991. Solexa modified these to incorporate a cleavable fluorescent label.
That leaves one final missing piece, cluster generation. Without an amplification approach Solexa would have had to use single molecule detection. Single molecule detection was significantly harder in the late 1990s. And even now, single molecule sequencing shows error rates significantly higher than achievable with clonal approaches.
Solexa acquired this technology from Swiss startup Manteia Predictive Medicine for, at an estimate, 1.5M USD [1]. This is a shockingly low price for a technology now foundational to a billion dollar market… but reports suggest that Manteia owner Serono had lost strategic interest in sequencing, which may explain the low acquisition price.
What was Manteia?
Manteia was a spin out of Serono, founded by Dr Pascal Mayer. They appear to have been pretty forward thinking, and were part of a small crop of next-gen sequencing startups that appeared in the late 1990s (there are now over 40).
Most of the technical information here, comes from a couple of presentations on slideshare [2] [3]. But there’s also ample information in their patent filings.
The basic approach suggested by Manteia, is pretty familiar, amplify single molecules on a solid surface, and sequence.
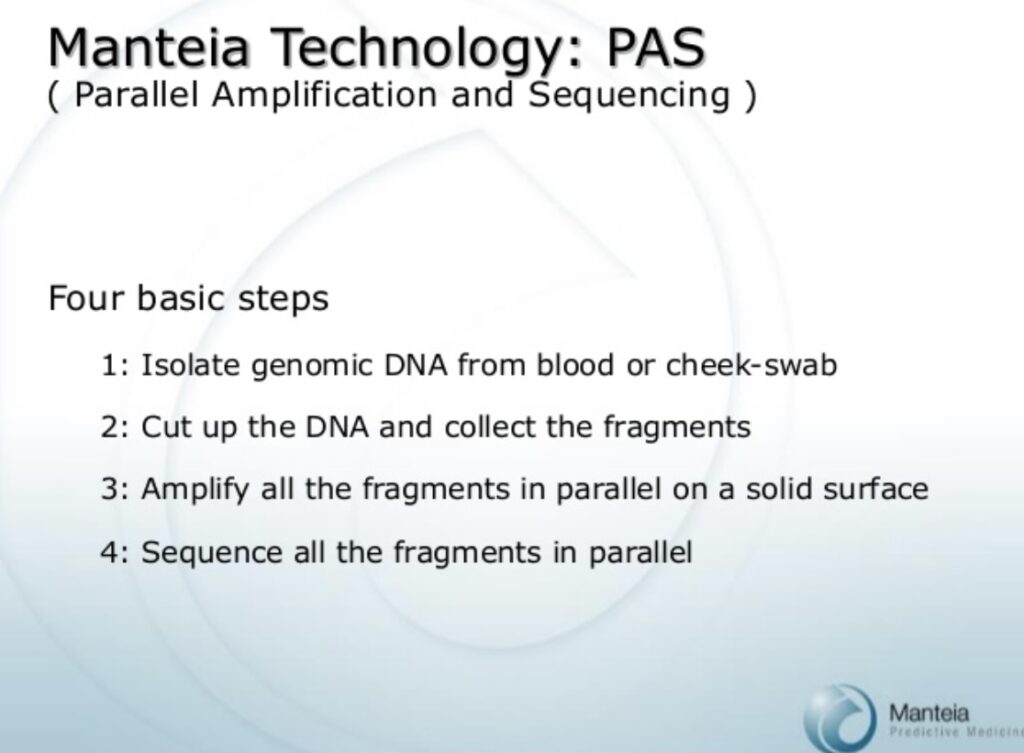
And indeed, the bridge amplification approach described, closely matches that presented by Illumina today:
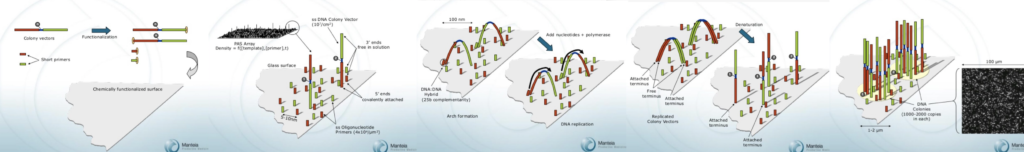
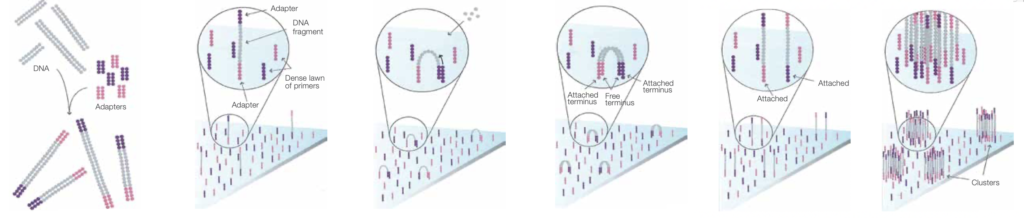
The image also very look very much like those from early genome analyzer 1 and 2 instruments. In fact look pretty similar to today’s Miseq images. In fact, Manteia images look slightly cleaner. Reflect the fact they they used a single channel chemistry, showing no crosstalk.
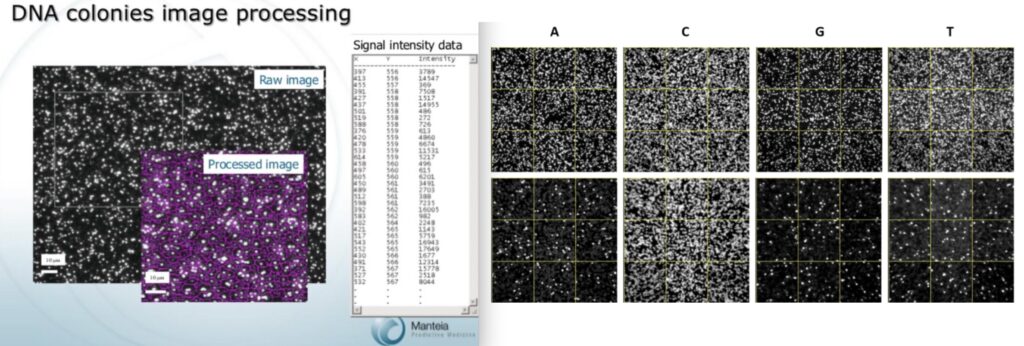
The Manteia sequencing approach was far different than that used by Solexa however. While Manteia were also proposing sequencing-by-synthesis, this presentation doesn’t show fluorophores being cleaved. In fact, it look like the dyes stay attached, and signal is ever increasing.
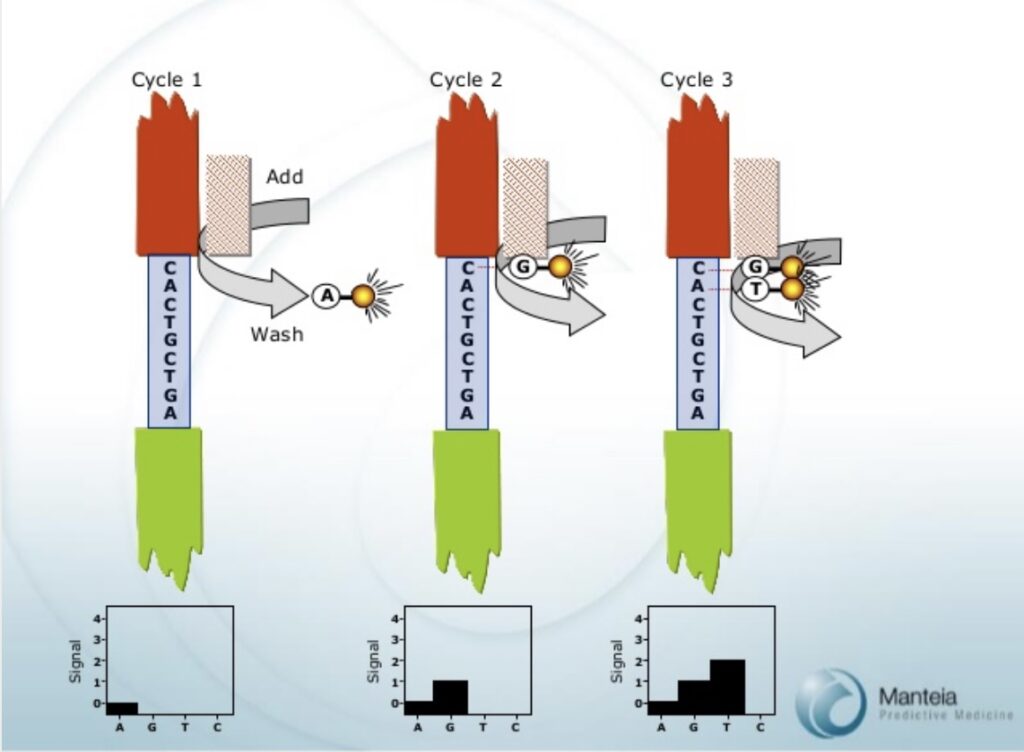
Manteia would therefore have had to detect stepwise increases in signal. The chemistry appears to lack reversible terminators. Homopolyers would therefore cause larger jumps in signal intensity. Precise determination of homopolymer length would probably have been difficult, a problem that still exists in some approaches (Ion Torrent).
As for Illumina sequencing, phasing (clusters getting out of sync as they fail to incorporate) would be an issue. But the lack of reversible terminators makes phasing errors harder to compensate for algorithmically.
Photobleaching, would effect not only the current base, but signals for all prior incorporations. This would need to be compensated for and could be another source of error.
Ultimately, an ever increasing “background” intensity would also limit read length.
Overall, these issues seem challenging. Switching this chemistry over to a reversible terminator/cleavable dye approach was likely key to Solexa’s development of a viable sequencing platform.
But Manteia do appear to have generated proof of concept sequencing results:
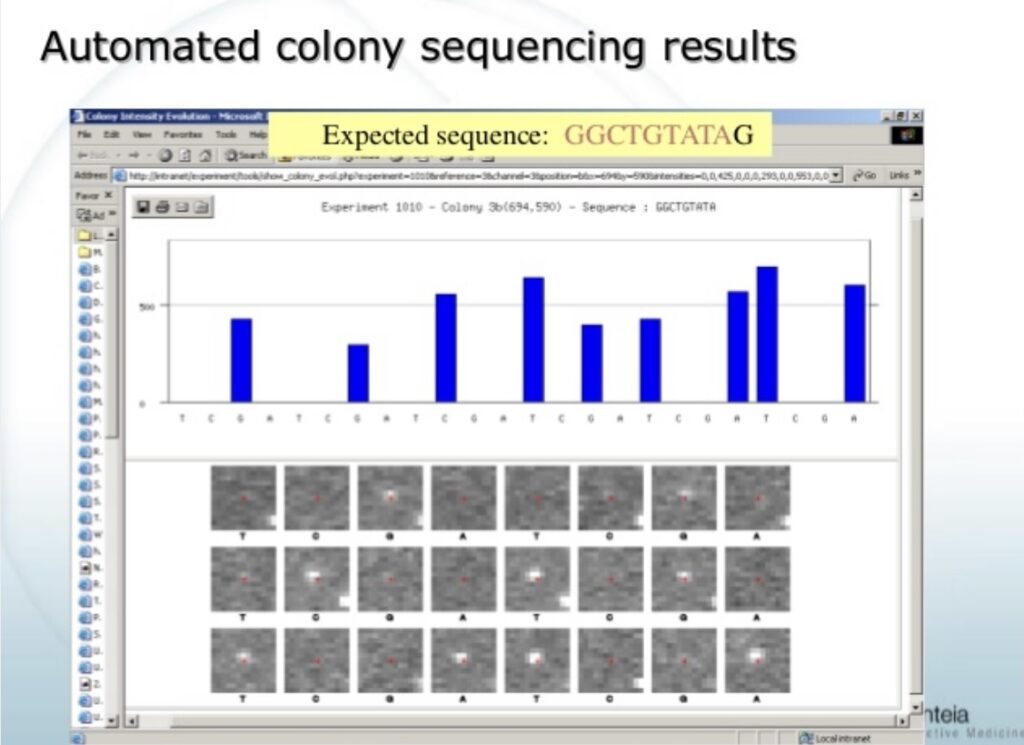
Unfortunately, we’ll never know how Manteia might have progressed the platform based on these early sequencing results. As development at Manteia stopped before the platform was fully realized.
What does this means for Illumina today?
Manteia initially reported cluster generation in 1998. The earliest IP I could find referring to the bridge amplification approach was from September 1998, and expired in 2020.
Illumina is currently using Solexa’s reversible terminator IP to block the sale of MGI instruments in the US. That IP appears to expire in 2024.
After this point, it seems likely that other players will be able to act on this IP and build essentially, Solexa-style SBS sequencers.
Current Illumina instruments have progressed somewhat. The 2-color sequencing, and exclusion amplification IP is still active as far as I’m aware.
However even without this IP, it’s likely that a respectable Illumina-clone could be assembled based on the original Solexa approach.
It therefore seems likely that new “Solexa-style” approaches will appear in the not too distant future. And at the very least, MGI will be able to sell instruments in the US again.
This broadening of the sequencing market is likely what’s driving Illumina push into diagnostic applications. Recent acquisitions of GRAIL, and Verinata Health give them a route into clinical diagnostics. Where they not only own the core sequencing technology, but the diagnostic application too. This allows them to take a larger share of the profit, and more closely lock-in a diagnostic customer base.
Notes/References
[1] Solexa’s 2004 accounts note that “These rights were purchased from Manteia SA under terms of a joint asset purchase agreement with Lynx Therapeutics”. Overall it looks likes the Manteia rights cost them somewhere in the region of 1.5M USD, that is unless payments from the recently merged Lynx side of Solexa hide additional payments.
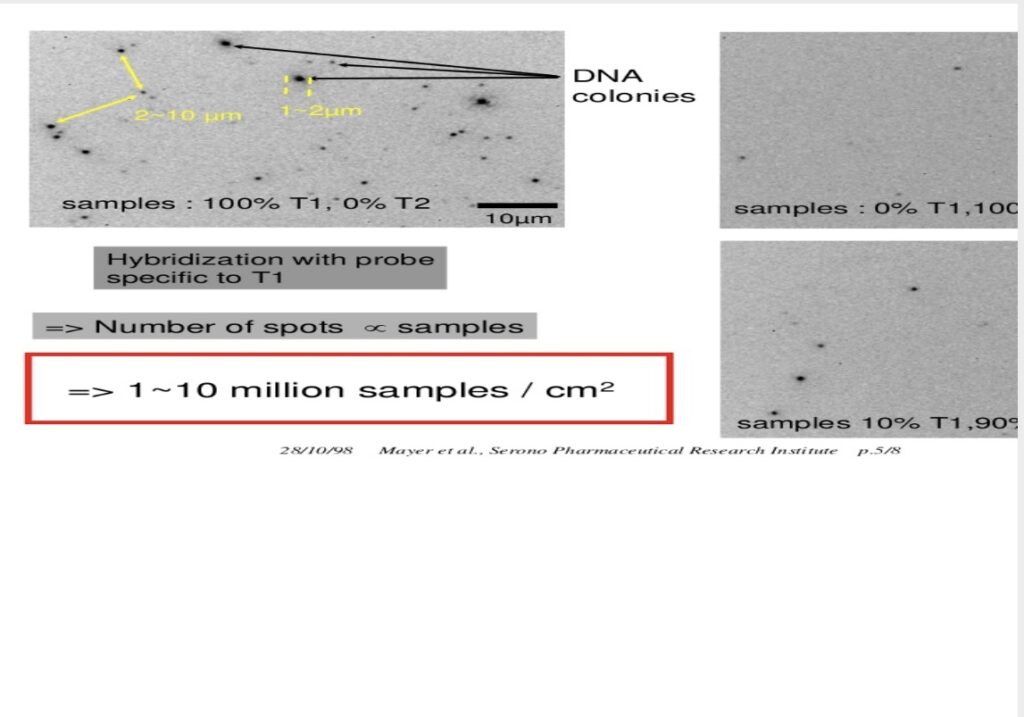
[2] “A very large scale, high throughput and low cost DNA sequencing method based on a new 2-dimensional DNA auto-patterning process”,P. Mayer, L. Farinelli, G. Matton, C. Adessi, G. Turcatti, J.J. Mermod, E. Kawashima, presented at the Fith International Automation in Mapping and DNA Sequencing Conference, St. Louis (MI,USA), October 7-10, 1998. Invited presentation (P. Mayer). Colonies from the 1998 presentation:
[3] A non confidential corporate presentation of “Manteia Predictive Médicine” as of September 2003. Présents DNA colony sequencing resutls, instrument, DNA preparation for genotyping.