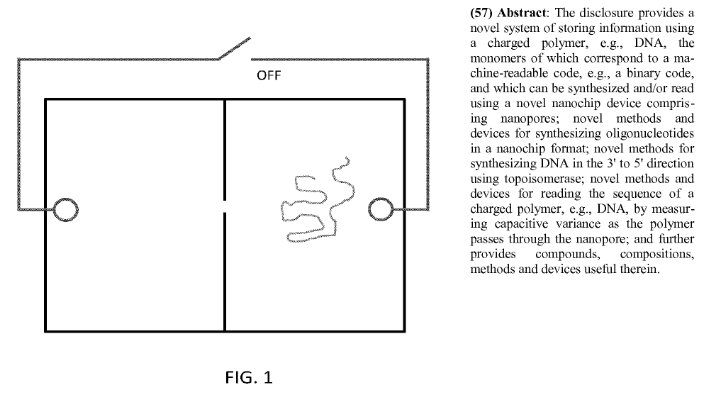
Read/Write DNA Devices
Yesterday I wrote up my notes on Iridia. I think one of the things I find so fascinating about the concept is the potential to create a system that can both read and write DNA. This move to read/write DNA devices seems like a step change from discrete sequencers and synthesizers.
To briefly recap, the Iridia system used nanopore to selectively expose DNA strands to enzymes. The enzymes can’t make their way through the pore, but you can drive the charged DNA strand through the pore under a bias voltage.
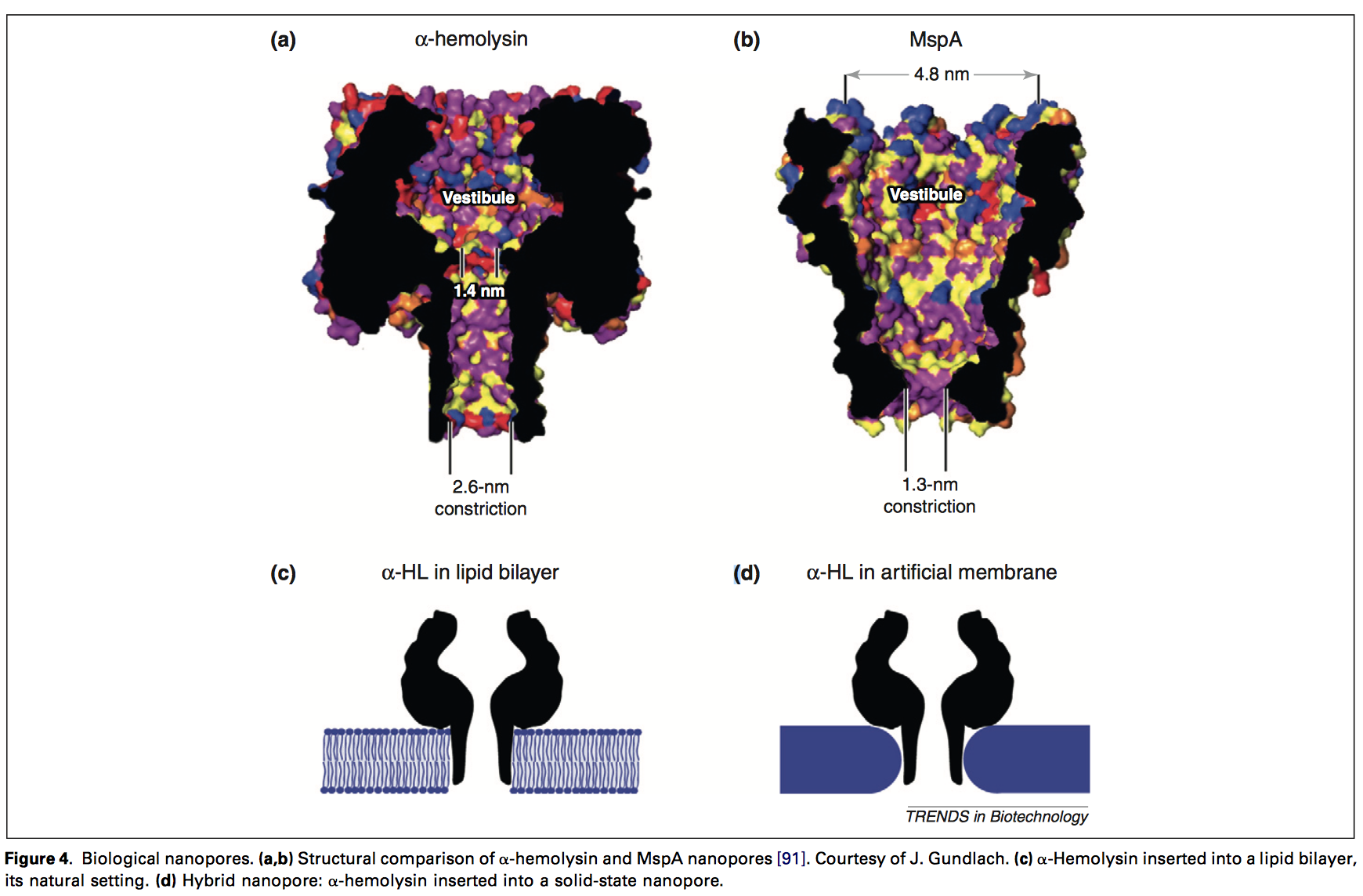
The use of a nanopore also clearly lends itself to using this same aperture for sequencing the DNA. This could be through the detection of the blockage of an Ionic current, or embedded electrodes (like some solid state nanopore systems). Either solid-state, or protein nanopores could be used. Apertures might even be constructed in other ways (like Armonica’s tortuous nanopores).
So potentially, you have a DNA synthesis platform, that can also QC the strand at every base incorporation as it passes through from one chamber to another.
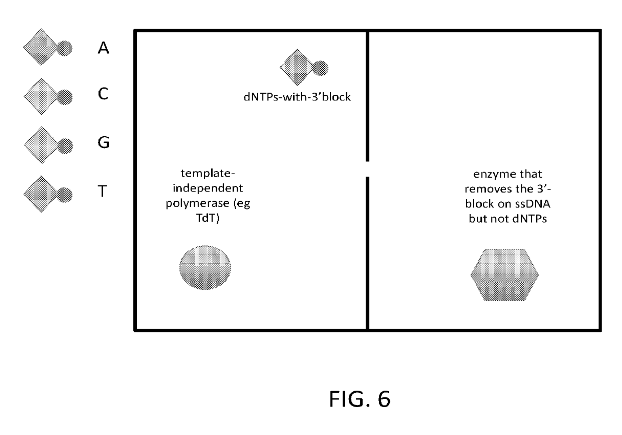
One issue with at least some embodiments of the Iridia concept from their patent, is that single nucleotides can make their way through the pore as well was the strand under synthesis. This means they need to remove the terminator from the base already incorporated into the strand only (I think they may have a way of doing this). I guess it can also increase the potential for misincorporation and might complicate the fluidic system.
However it feels like if the correct components can be assembled, you might be able to create a chip system that can read and write DNA and has no external fluidic components.
I thought it might be fun to play around with these ideas a bit…
The diagram below shows what this (Computer Scientist) imagines such a system might look like schematically:
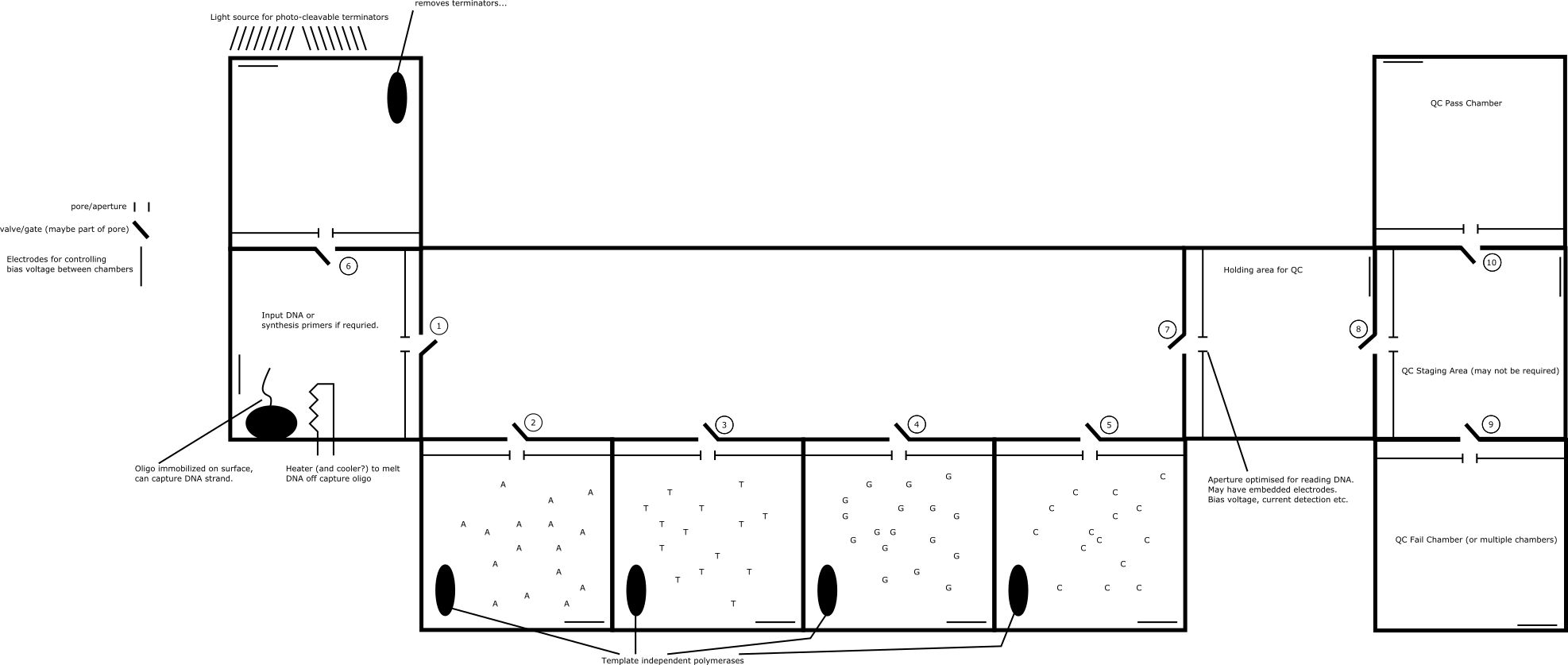
In the above diagram I’ve shown a system with multiple chambers. Each chamber has two apertures shown. One is a nanopore/nanoscale aperture through which only DNA can pass. The other is a valve, which stops all flow into the chamber. The valve can be much bigger than the nanopore, these are all normally closed. Potentially the valve could be part of the pore (for example a voltage gated ion channel [2]) or might not be required at all. But this valve could be large and should be easy to fabricate.
The strand under synthesis sits in the input DNA chamber. You then open the value leading out of this chamber (1) and going to one of the nucleotides, (2) for example. A bias voltage is applied between these chambers to drive the DNA into the correct nucleotide chamber. In this chamber there’s a template independent polymerase. The nucleotides in this chamber are terminated, such that only a single nucleotide is incorporated into the strand.
The bias voltage is then reversed, and the strand flows back into the “input” chamber. A number of single nucleotides come along for the ride. However in the input chamber the strand is captured. This could be by hybridising to an immobilized complementary strand, though there might be other methods. With the strand captured you reverse the bias voltage again and the single nucleotides flow back into their original chamber. You can then close the valves to keep them contained. The strand is released by heating the strand, melting it.
Next you need to cleave the terminator. This will depend on the type of terminator used. One possibility might be to use photo-cleavable terminators such as those developed by LaserGen [1]. If this is the case, then you would just need to turn on a light source to remove the terminator. Another chamber could be used however, particularly if there is some other process (perhaps there’s an enzymatic process?) that is used to remove the terminator.
The process would then continue as above cyclically. Depending on the quality of the pores/apertures, you can also measure the ionic current as the strand passes through the pore. This may be sufficient to determine the sequence during synthesis.
In any case, once synthesis is complete you can open another valve (7) and set bias voltages between the chambers (input and holding for QC chambers) such that the DNA will flow through an exit pore. This pore could be specifically designed for sequencing and might have additional embedded electrodes to enable this.
You could also QC the strand (did synthesis complete correctly?) and sort the strand accordingly.
Of course, the same system could also be used as a read system only, just insert complete strands at the start of the process.
Potentially all the nucleotides and reagents could come preloaded, giving a simple, almost solidstate system. While initially it might be desirable to fabricate only certain parts of the system using nanofabrication, ultimately it might be possible to integrate all components onto a single chip.
Notes
[1] https://webcache.googleusercontent.com/search?q=cache:hkUhGFAhmmgJ:https://www.genomeweb.com/sequencing/lasergen-says-its-new-reversible-terminators-could-improve-several-sequencing-pl+&cd=15&hl=en&ct=clnk&gl=jp
[2] https://en.wikipedia.org/wiki/Voltage-gated_ion_channel